MesoPher, Amphera’s lead product, is comprised of autologous DCs (i.e. from the patient) loaded with “PheraLys” – Amphera’s allogeneic lysate of mesothelioma cell lines.
Dendritic cells can be generated ex vivo from monocytes obtained from the patient’s peripheral blood, outside the immunosuppressive environment. DCs can be loaded ex vivo with TAAs. These may be synthetic peptides, mRNA coding for parts of TAAs, a lysate of autologous tumour material (i.e. from the patient) or an allogeneic tumour cell lysate.
An allogeneic tumour lysate has several advantages, as this is an off-the-shelf source of various TAAs that can be shared across different tumour types, it eliminates the need for obtaining autologous tumour material, a known major logistical hurdle, and it provides treatment standardization across patients. Also, the use of lysate containing a broad repertoire of TAAs may avoid tumour-immune escape which has been described for single-TAA strategies.
The allogeneic lysate PheraLys is produced from a number of clinical grade mesothelioma cell lines. These cell lines have been extensively tested and characterized. The cell lines are stable and ensure an inexhaustible source of tumour cell derivatives of constant quality. PheraLys, the allogeneic lysate from these cell lines, will be used in dendritic cell therapy for pancreatic cancer, mesothelioma and other cancers.
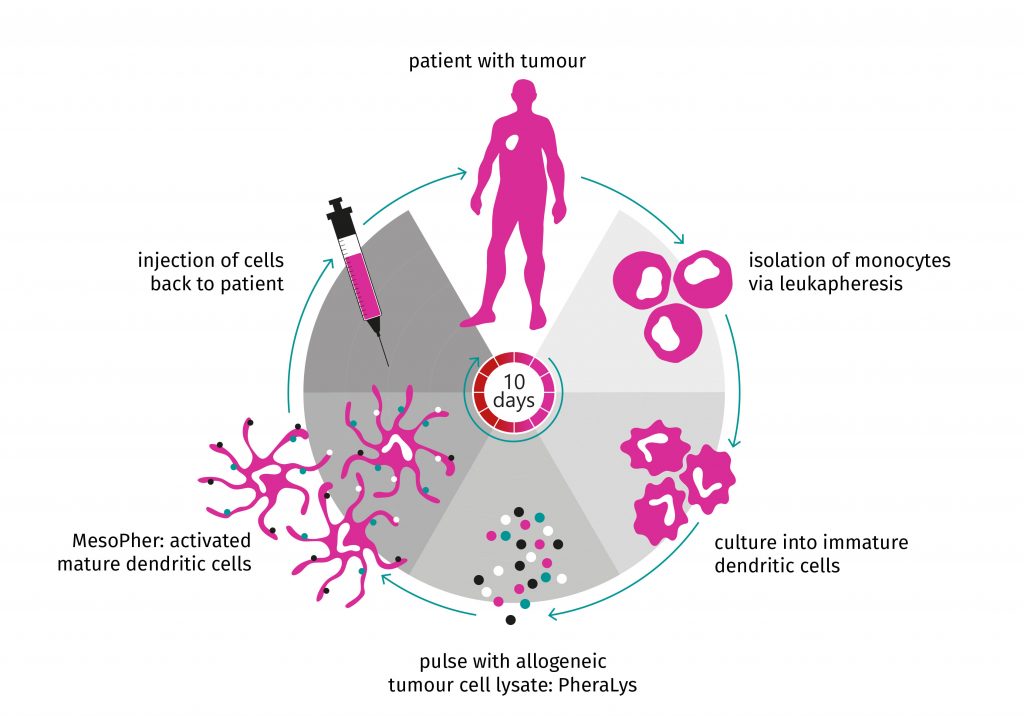
MesoPher, autologous DCs loaded with PheraLys, is a personalized cellular product that is prepared for every individual patient. It is an Advanced Therapy Medicinal Product (ATMP). A robust, Good Manufacturing Practice (GMP) compliant production process including all relevant documentation is established and operational. The infographic below illustrates the straightforward steps to produce MesoPher for a certain patient.
Blood from a patient is collected via leukapheresis. This is a routine, standardized hospital procedure. From the leukapheresis product, monocytes are isolated and differentiated ex vivo (=outside the body) into immature DCs. These autologous (=from the patient) dendritic cells are loaded ex vivo with PheraLys, our proprietary allogeneic mesothelioma tumour cell lysate. Finally, the patient is treated with MesoPher, his/her own activated, mature dendritic cells.